Definition
- J Obstet Gynaecol Can. 2010 May;32(5):423-5, 426-8.
- Nat Rev Endocrinol. 2011 Apr;7(4):219-31.
PCOS is characterized by hyperandrogenism, irregular ovulatory cycles, and a metabolic derangement including glucose intolerance and hyperinsulinemia. Manifestations of PCOS are heterogeneous, and several consensus definitions of the disorder have been produced to describe the disease, with various emphases on clinical or biochemical hyperandrogenism, polycystic ovaries, and oligoanovulation. The Rotterdam Consensus (2003) defines PCOS as at least two of the following characteristics:
- Clinical hyperandrogenism and/or hyperandrogenemia
- Oligoanovulation
- Polycystic ovaries on ultrasound
Although other definitions emphasize the presence of clinical or biochemical hyperandrogenism as an important characteristic of the disease, there is some debate as to the centrality of hyperandrogenism in PCOS. The more inclusive Rotterdam criteria may be appropriate in patients where clinical hyperandrogenism is difficult to assess.
Pathogenesis and risk factors
- J Obstet Gynaecol Can. 2010 May;32(5):423-5, 426-8.
- Nat Rev Endocrinol. 2011 Apr;7(4):219-31.
- N Engl J Med. 2005 Mar 24;352(12):1223-36.
|
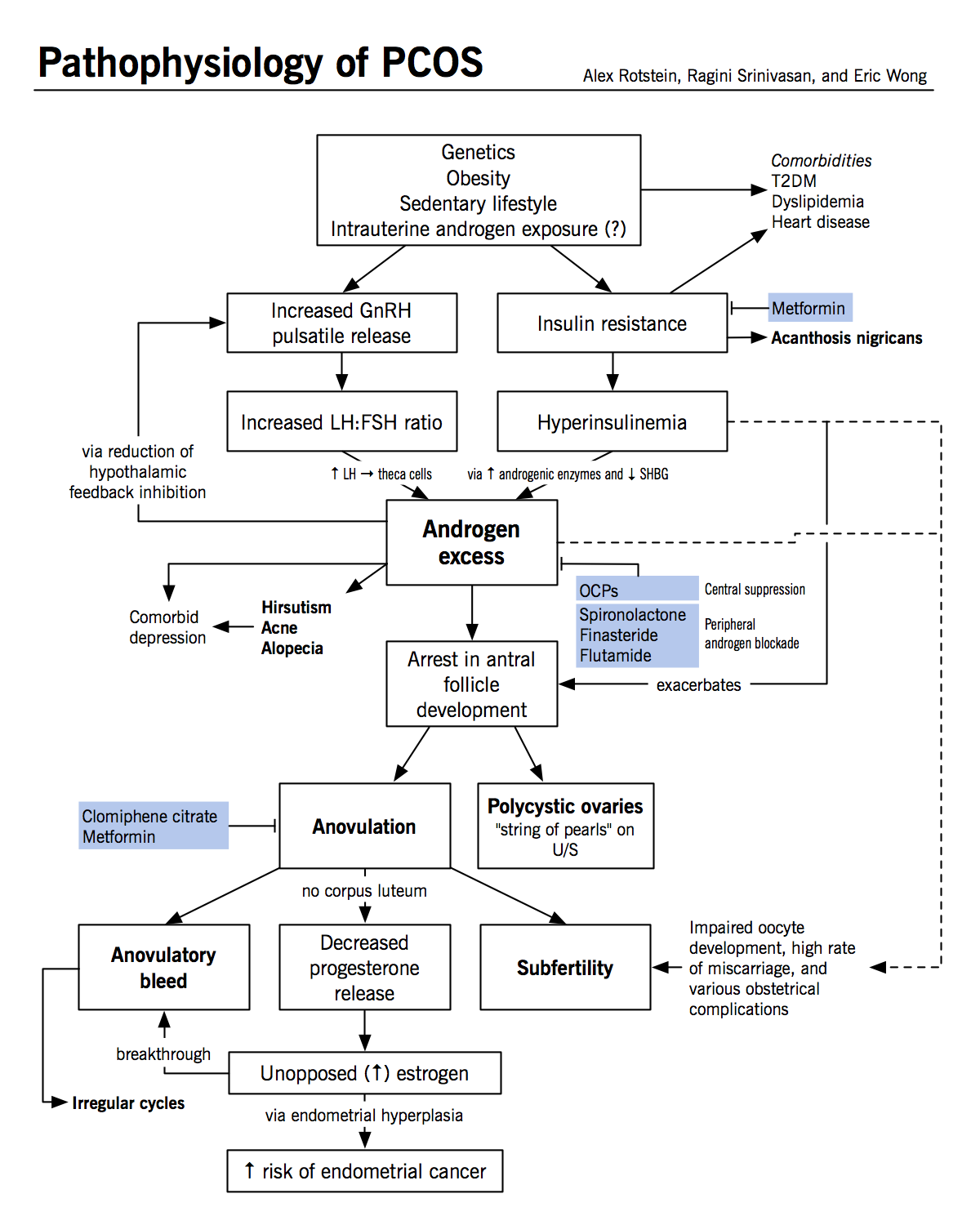
Genetics: PCOS is believed to be a complex disorder, with genetic as well as environmental factors contributing to development of the disease. |
20-40% of female first-degree relatives of women with PCOS also have the syndrome, suggesting that the disease is partially heritable and clusters in families. Prevalence and severity of presentation vary with ethnicity, with South Asians at a higher risk of disease. Some candidate genes have been identified as contributing to risk of the disease, including 7β-hydroxysteroid-dehydrogenase type 6 (HSD17B6). |
|
Intrauterine exposures: exposures to testosterone in utero may predispose to the later development of PCOS. |
Animal studies have demonstrated that in utero exposure is correlated with development of a PCOS-like syndrome including hyperinsulinemia, hyperandrogenism, oligoanovulation, and polycystic ovaries. Exposure to androgens may impair estrogen and progesterone inhibition of GnRH, contributing to increased pulse frequency. |
|
Environment/lifestyle: several lifestyle factors and environmental exposures have been associated with a more severe PCOS phenotype. |
Sedentary lifestyle is associated with increased metabolic dysfunction, and weight gain is associated with oligoanovulation and hyperandrogenism. BPA and other environmental androgen-disrupting chemicals may accumulate to a greater extent in individuals with PCOS because of decreased hepatic clearance; these also induce androgen production and insulin resistance. |
|
Obesity: although obesity is not believed to cause PCOS, it is known to exacerbate the symptoms of the disease. |
Obesity is present in 30-75% of women with PCOS. Adipose dysfunction contributes to the development of glucose intolerance and hyperinsulinemia, which in turn can exaggerate the manifestations of hyperandrogenism (see below). Obese women with PCOS are at increased risk of anovulation and consequent subfertility. |
Pathophysiology
- J Obstet Gynaecol Can. 2010 May;32(5):423-5, 426-8.
- Nat Rev Endocrinol. 2011 Apr;7(4):219-31.
- N Engl J Med. 2005 Mar 24;352(12):1223-36.
Hyperandrogenism
- Hyperandrogenism is the most characteristic feature of PCOS, and some argue that it is the defining feature of the disease
- Hyperandrogenism is exacerbated by hyperinsulinemia and antral follicle arrest and may itself increase the risk of follicle arrest
- Similar ovarian characteristics have been noted in women with other conditions of androgen excess such as congenital adrenal hyperplasia
Neuroendocrine abnormalities
- Women with PCOS have an increase in the frequency of GnRH pulses; shorter pulses preferentially promote the production of luteinizing hormone (LH) and result in a decrease in the production of follicle stimulating hormone (FSH)
- Patients with PCOS often exhibit an increase in the LH:FSH ratio, which may contribute to the ovarian excess of androgens relative to estrogens
- It is unclear if patients with PCOS have an intrinsically faster GnRH pulsation mechanism which initiates hyperandrogenism in the ovaries, or if oligoanovulation itself promotes more rapid pulsations in GnRH via a reduction in circulating progesterone
- Normally, progesterone is released from the corpus luteum following ovulation
- Progesterone acts to slow GnRH pulsation
- In PCOS, a decrease in ovulatory events may cause a decrease in circulating progesterone
- Exposure to androgens in utero or prepubertally may decrease the inhibitory effects of estrogen and progesterone on the hypothalamus and contribute to increased pulsatility
Insulin resistance and T2DM
- 50-70% of patients with PCOS exhibit metabolic abnormalities, including poor glucose tolerance and hyperinsulinemia
- This is not solely a consequence of increased visceral obesity; rather, obesity and hormonal abnormalities are thought to make additive contributions to insulin resistance:
- Patients with PCOS exhibit a greater degree of insulin resistance than patients with the same BMI and visceral adiposity who do not have PCOS
- Functional insulin resistance is considered a consequence of defects in insulin-mediated glucose transport and signaling in adipocytes and myocytes; this may be the result of a dysregulation in adipokine production and signaling from adipose tissues but the mechanism is incompletely understood
- The resulting hyperinsulinemia leads to insulin spillover into other tissues, most commonly the skin. Insulin acts via insulin-like growth factor receptors to cause excess keratinocyte growth, producing velvety skin patches known as acanthosis nigricans.
Polycystic ovaries
- Polycystic ovaries are present in 20-30% of women and are not essential for the diagnosis of PCOS
- The “cysts” in polycystic ovaries are not true cysts, but rather antral follicles which have arrested in development
- This is thought to occur because of hormonal abnormalities:
- Hyperandrogenism: arrest occurs when the granulosa cells of the ovaries normally begin to produce estrogen by aromatizing androstenedione produced by the theca cells; excess 5a-reduced androgens in the ovaries are thought to inhibit the action of aromatase and therefore reduce estradiol synthesis, which is required for further maturation
- Hyperinsulinemia: exacerbates ovarian hyperandrogenism by (1) increasing 17a-hydroxylase activity in theca cells and promoting androstenedione and testosterone production; (2) promoting LH- and IGF1-stimulated androgen production; and (3) elevating free testosterone by decreasing the production of sex hormone binding globulin (SHBG)
Long-term morbidity
- Subfertility: this is largely a consequence of oligoanovulation, but may also result from abnormalities in oocyte development due to hormonal or other abnormalities
- Miscarriage: there is an increased risk of miscarriage in PCOS patients who do conceive; however, this risk is confounded by the high rate of obesity in this population, which is also a risk factor for miscarriage
- Cardiovascular disease: patients with PCOS often exhibit dyslipidemia, which is likely related both to hyperinsulinemia and hyperandrogenism
- T2DM: patients with PCOS are thought to have an increased risk of developing T2DM above the risk conferred by their level of insulin resistance and as many as 10% may develop T2DM by their fourth decade
- Malignancies: a combination of hyperinsulinemia, hyperandrogenism, and oligoanovulation increases the risk of endometrial cancer and other endometrial disorders
- Psychiatric disorders: women with PCOS have an increased risk of anxiety, depression, binge-eating disorder, and bipolar disorder
Clinical features
- JAMA. 2007 Feb 7;297(5):509-19.
- Nat Rev Endocrinol. 2011 Apr;7(4):219-31.
- N Engl J Med. 2005 Mar 24;352(12):1223-36.
- Manifestations of PCOS are varied, but many signs of PCOS are intimately related to disease pathophysiology
- Hyperandrogenism:
- Hirsutism
- Acne
- Alopecia: male-pattern hair loss
- Hyperinsulinemia:
- Acanthosis nigricans
- Hyperandrogenism:
- A significant number of patients with PCOS are also obese (30-75%); obesity is thought to exacerbate the symptoms of hyperandrogenism and hyperinsulinemia
- Patients may not exhibit frank signs of hyperandrogenism or hyperinsulinemia, and their clinical history must be weighed along with their physical and biochemical signs
Treatment
- J Obstet Gynaecol Can. 2010 May;32(5):495-502.
- Nat Rev Endocrinol. 2011 Apr;7(4):219-31.
|
Lifestyle modification: may help attenuate all symptoms of PCOS and reduce the long-term risk of infertility, CVD and T2DM. |
This is the first line of PCOS management. Increased exercise, improved diet, and weight loss can help to reduce the metabolic abnormalities associated with PCOS. Weight loss of as little as 5-10% has been demonstrated to correct oligoanovulation and improve the ability of women with PCOS to conceive. |
|
Estrogen and progestin oral contraceptive (OCP) therapy: treatment of acne, hirsutism and irregular menstrual cycles. |
Can be used to normalize androgen levels and attenuate the signs of hyperandrogenism as well as to regulate menstrual cycles. This also helps to reduce the risk of heavy and irregular menstrual bleeding associated with the loss of normal estrogen and progestrone levels. |
|
Anti-androgens (e.g. spironolactone, finasteride, flutamide): treatment of acne and hirsutism. |
Spironolactone and flutamide competitively inhibits DHT and testosterone by binding to their receptors in peripheral cells (e.g. hair follicles). Finasteride is a 5a-reductase inhibitor that inhibits conversion of testosterone to the more potent DHT in peripheral cells. Anti-androgens can be used synergistically with OCPs, which act centrally to suppress androgen release. Note that anti-androgens are contraindicated in pregnancies because they are teratogens. |
|
Metformin: treatment of glucose intolerance, hyperinsulinemia, and anovulation. Reducing circulating insulin levels may secondarily reduce ovarian androgen synthesis. |
Metformin reduces glucose intolerance and hyperinsulinemia by increasing insulin sensitivity and decreasing hepatic gluconeogenesis and lipogenesis; it can therefore be used to help prevent and treat T2DM. Treating these factors can also induce ovulation. Combined treatment with metformin and clomiphene citrate (see below) has been shown to be more effective than either agent alone in inducing ovulation. |
|
Clomiphene citrate: for inducing ovulation. |
Clomiphene citrate is a selective estrogen receptor modulator (SERM). It induces ovulation by interfering with estrogen feedback to the brain and thus increasing FSH release. There is increased risk of multigestational pregnancy (e.g. twins or triplets) because of the large number of antral follicles in polycystic ovaries. Furthermore, although most women will ovulate on clomiphene citrate, only half will actually conceive. This may be related to the anti-estrogenic effects of clomiphene, which can result in thinning of the endometrium. Clomiphene citrate treatment should be limited to 12 cycles because longer-term treatment is associated with increased risk of ovarian cancer due to ovarian hyperstimulation. |
|
Gonadotropin therapy: recombinant FSH and hCG can be used to induce ovulation in cases where treatment with clomiphene citrate and metformin has been unsuccessful. |
Exogenous gonadoptropins can be administered to mimic physiological mechanisms of follicle development. FSH is given to promote growth of a dominant follicle to a particular size, and then human chorionic gonadotropin is used to induce ovulation. This therapy must be closely monitored with imaging and laboratory studies to minimize the risks of multigestational pregnancy and ovarian hyperstimulation. |
|
Ovarian drilling: a laparoscopic surgical procedure that may be used to treat clomiphene citrate-resistant anovulation. |
Ovarian drilling involves the creation of ~10 perforations in the ovary using either cautery or laser. The ablation of some of the ovarian theca is thought to help induce ovulation by decreasing androgen production. This procedure may have similar efficacy to gonadotropin therapy, but surgical complications such as adhesion formation remain a concern. This procedure is especially useful in patients with other existing indications for laparoscopy. |
|
IVF: used for the treatment of infertility in women who have not responded to other therapies to induce ovulation. |
IVF involves the retrieval of oocytes from the ovaries and in vitro combination with sperm to produce embryos. Viable embryos are then transferred into the uterus. Women with PCOS have similar success and live birth rates compared to women without PCOS. Risks of the procedure include multigestational pregnancy because of the transfer of multiple embryos and ovarian hyperstimulation as a consequence of gonadotropin therapy, which is used prior to oocyte retrieval to promote follicular development. |