Definition
Lancet. 2012 Apr 7;379(9823):1341-51.
Am J Respir Crit Care Med. 2007 Sep 15;176(6):532-55.
COPD is a characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and lung to noxious particles or gases.
[sidebox title=”Asthma vs COPD” content=”Asthma = fully reversible airway narrowing
COPD = not fully reversible airway narrowing” display=”inline” float=”right”]
COPD can be divided into 2 clinical phenotypes: emphysema and chronic bronchitis.
- Emphysema is defined pathologically as enlargement of distal air spaces.
- Chronic bronchitis is defined clinically as cough productive of sputum occurring on most days in 3 consecutive months over 2 consecutive years.
Etiology
- Lancet. 2012 Apr 7;379(9823):1341-51.
- Am J Respir Crit Care Med. 2007 Sep 15;176(6):532-55.
- Lancet. 2009 Aug 29;374(9691):733-43.
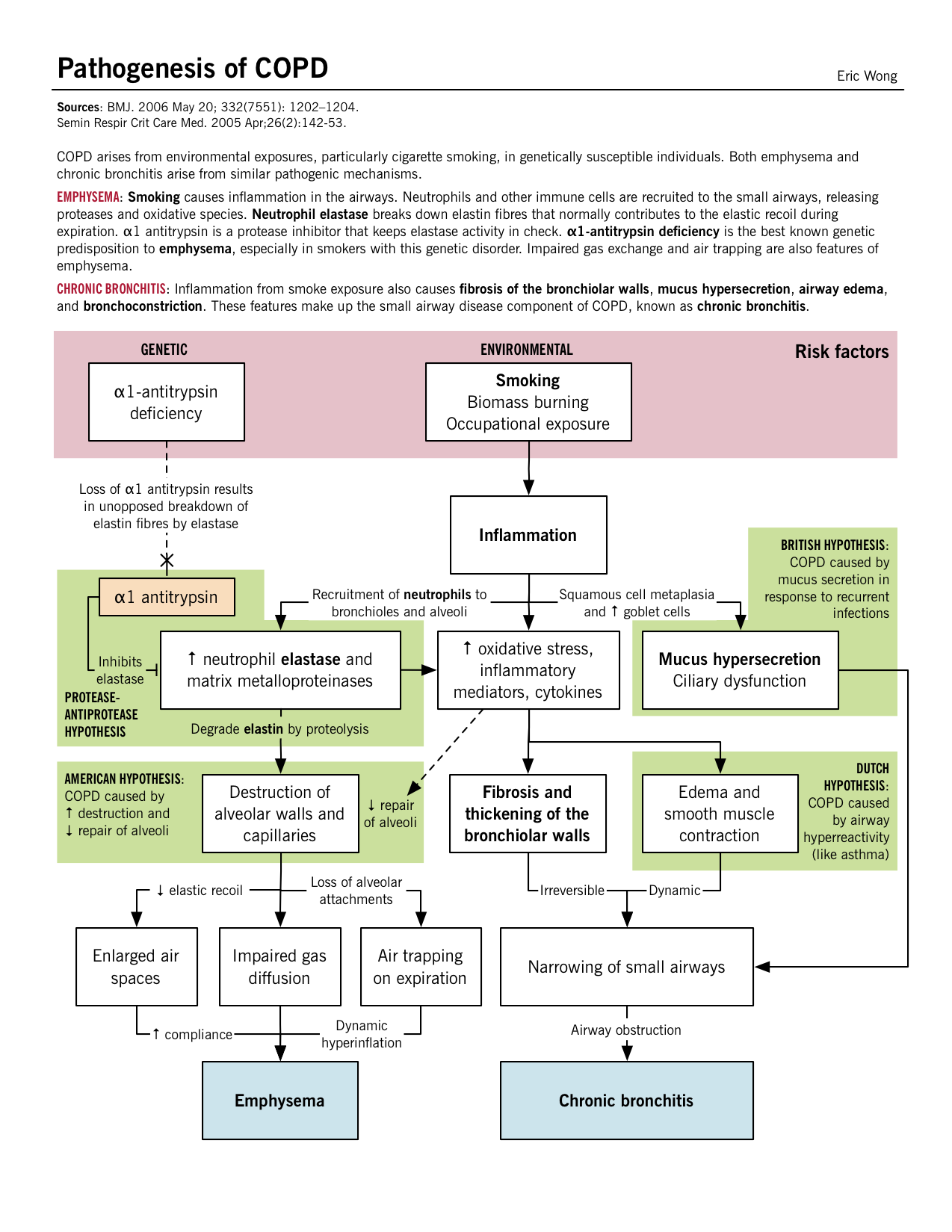
Many factors contribute to the development of COPD, including genetic factors like alpha1-antitrypsin deficiency, occupational exposures to dusts and chemicals, pollution, respiratory infections in childhood and cigarette smoke.
| Etiology | Mechanism(s) |
| Cigarette smoke | Presence of smoke particles in the lungs leads to an inflammatory response with increased macrophage and neutrophil infiltration into the lungs. These immune cells release cytokines, chemokines and elastases, which damages the lung parenchyma over time. |
| Occupational exposures to dust and chemicals | Etiology unclear, however, is hypothesized to be a similar inflammatory response that damages the alveoli. |
| Alpha-1 antitrypsin deficiency | Alpha-1 antitrypsin is a serine protease inhibitor (SERPIN) secreted by the liver into the blood which inhibits the enzyme neutrophil elastase from damaging the lung tissue. Deficiency of this alpha-1 antitrypsin leads to unopposed elasteolysis (destruction of the elastin fibers in alveolar walls) and development of early emphysema. This is the protease-antiprotease hypothesis of emphysema development. |
| Chronic IV drug use | IV drug users of cocaine, methadone and heroin are at higher risk for developing COPD; this is attributed to the vascular damage induced by the insoluble filler (cornstarch, cellulose, talc, fiber etc) found in IV drugs. |
Pathogenesis, pathophysiology and clinical features
BMJ. 2006 May 20; 332(7551): 1202–1204.
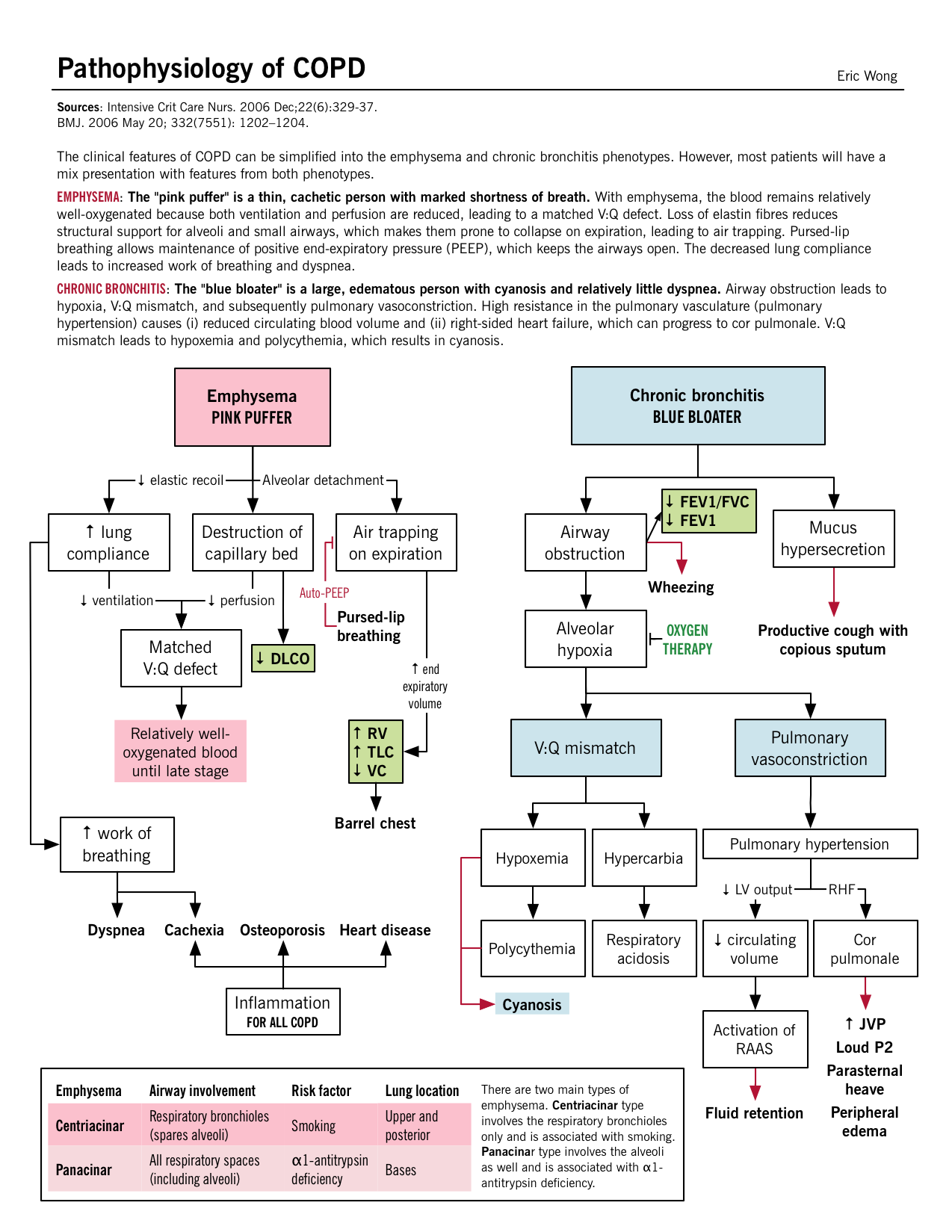
Though a breakdown of COPD into emphysema and chronic bronchitis is helpful, typically patients have features and findings of each and cannot be simply classified.
| Emphysema | Chronic bronchitis | |
|
Pathogenesis Am J Respir Cell Mol Biol. 2005 May;32(5):367-72. |
The inflammatory response, mediated by neutrophils, macrophages and CD8+ T-cells, release inflammatory mediators and enzymes that damage the lung parenchyma. Proteases like elastase and matrix metalloproteinases (MMPs) released by these inflammatory cells break down the connective tissue of the alveolar walls and the septae. A loss of elastic recoil leads to diminished expiratory flow rates, air trapping and airway collapsing. | Mucous gland enlargement, goblet cell hyperplasia and mucociliary dysfunction occur in larger airways, causing excessive mucus production and build-up reducing the airway lumen.Although these pathological changes in the large airways, it appears that the major site of increased airway resistance is the small airways (≤ 2mm). Fibrosis and smooth muscle hypertrophy may occur along with excess mucus production and cellular infiltration in the peripheral airways. |
|
Pathophysiology BMJ. 2006 May 20; 332(7551): 1202–1204. |
Parenchymal destruction: Recurrent damage to the alveoli eventually leads to septal destruction along with the capillary bed also. Matched V/Q defect: Since both the terminal bronchioles and alveoli along with the capillary bed have been destroyed, a matched defect exists between the ventilation and perfusion; areas of low ventilation also have poor perfusion. Mild hypoxia: Despite the “matched” V/Q defect, overtime hyperventilation develops and cardiac output (CO) drops which leads to areas of poor blood flow in relatively well oxygenated areas. Due to this poor CO, the rest of the body suffers from tissue hypoxia. Cachexia: At the pulmonary level, the low CO leads to pulmonary cachexia; which induces weight loss and muscle wasting. This gives these patients the characteristic “pink-puffer” appearance. |
Small airway inflammation: Mechanisms discussed above lead to inflammation in the smaller bronchioles and mucus secretions further narrow the airway lumen. Despite this, the parenchyma are relatively less damaged. V/Q mismatch: The physiologic response leads to a drop in ventilation and compensation with the rise in CO. Increased perfusion in the areas of poor ventilation takes place eventually causing hypoxia and secondary polycythemia. Severe hypoxia and hypercarbia: Chronic V/Q mismatch leads to decreased oxygenation/deoxygenation of the blood resulting in hypoxemia and increased CO2 retention (respiratory acidosis ensues). Pulmonary hypertension and cor pulmonale: Chronic hypercapnia and respiratory acidosis lead to arterial vasoconstriction in the lungs. With the retrograde pressure build-up, the right ventricular pressures continue to rise and eventually causing RV failure. Otherwise, known as cor pulmonale. |
| Clinical signs/symptoms |
“Pink puffer” – type A Severe constant dyspnea/tachypnia (“puffing”): Likely related to increasing end-expiratory volume (decreased recoil), making each breath less efficient. Patients use accessory muscles (tripod position) and breath faster (hyperventilation) to compensate for feeling of inadequate ventilation. Dyspnea is also related to respiratory muscle fatigue from increased use as well as the flattening of the diaphragm which impairs its function. Mild cough: Irritation of the smaller airway can lead to the production of cough. Non cyanotic (“pink”): Matched V:Q defect; no hypoxemia. Thin/cachexic: Loss of skeletal muscle and subcutaneous fat due to inadequate oral intake as well as high levels of inflammatory cytokines (TNF-α) that cause such wasting. Diminished breath sounds on auscultation: Hyperinflation of alveoli and destruction of alveolar architecture causes decreased airway resistance. |
“Blue bloater” – type B Copious sputum production: High amount of sputum produced by the goblet cells. See Pathogenesis above. Cough: Irritation of the cough receptors, by the mucous, in the smaller and the large airways. Cyanotic (“blue”): The mismatched V/Q defect leads to inadequate oxygenation of the blood; most prominent in the lips and the nail beds. Volume overload (“bloater”): Most likely from from the right ventricular (RV) failure, known as cor pulmonale. Wheezy on auscultation: Due to airway obstruction. Compared to asthma, there is less bronchospasm and more mucus/hypertrophy in COPD. Rhonchi is a gurgling sound that may be heard due to mucus hypersecretion in the airways. |
Exacerbations
N Engl J Med. 2012 Jul 26;367(4):340-7.
Several studies have shown some link between bacterial colonization of the upper and the lower airways of patients and acute exacerbations of COPD. Furthermore, exacerbations seem to coincide with the rise in acute respiratory viral infections (influenza, parovirus etc). The pathogens introduce new antigens into the airways and the parenchyma which induces the secretion of chemokines (TNF-α and IL-6, IL-8 etc) and leukotrienes, both by the airway macrophages as well as the epithelium, which recruit neutrophils. These neutrophils, along with other immune cells secrete proteases and other media that further inflame the airways and destroy bronchial epithelium, leading to a clinical exacerbation.
Treatment
- Am J Respir Crit Care Med. 2013 Feb 15;187(4):347-65.
- Lancet. 2012 Apr 7;379(9823):1341-51.
- N Engl J Med. 2012 Jul 26;367(4):340-7.
Smoking cessation
Smoking cessation significantly slows progression of lung function decline and reduces mortality by 18%. It is the single most effective and important intervention in COPD.
Bronchodilators
Inhaled β2-agonists (e.g. short-acting salbutamol or long-acting salmeterol) act on β2-receptors on smooth muscle cells to cause bronchodilation. Inhaled anticholinergics (e.g. short-acting ipratropium or long-acting tiotropium) act to block acetylcholine’s effect on muscarinic receptors on smooth muscle cells, allowing bronchodilation. Inhaled β2-agonists and anticholinergics are used for both symptomatic management, as well as acute exacerbations of COPD. Long-acting bronchodilators are preferred over short-acting ones because of fewer doses and improved symptom management. For more effective treat of stable COPD, combination therapy using an inhaled β2-agonist and an anticholinergic can also be used.
Corticosteroids
Steroids suppress the inflammatory response by inhibiting transcriptions factors, including nuclear factor-κB, that regulate the transcription of various cytokines, chemokines, adhesion molecules, and other proteins that induce and perpetuate inflammation. Inhaled corticosteroids are not used for the treatment of COPD exacerbations; however, have been used in the long-term treatment of COPD in a minority of patients with stable COPD who demonstrate frequent exacerbations and bronchodilator reversibility. Systemic corticosteroids are recommended to be used during an acute exacerbation requiring hospitalization.
Oxygen
Cochrane Database Syst Rev. 2005 Oct 19;(4):CD001744.
Oxygen therapy is frequently provided along with pharmacological interventions to treat underlying hypoxemia in COPD patients. By reducing hypoxia in the alveoli, pulmonary vasoconstriction is reduced. Reducing pulmonary hypertension lowers right heart afterload, and improves right heart systolic function. Oxygen also reduces hypoxemia in the blood, which reduces the risk of developing polycythemia. However, oxygen therapy has only been shown to reduce mortality in those with severe hypoxemia (PaO2 < 55mmHg); otherwise there is no mortality benefit.
Pulmonary rehabilitation
Chest. 2007 May;131(5 Suppl):4S-42S.
The goal of pulmonary rehabilitation is to improve quality of life and daily functioning in COPD patients. Through a multidisciplinary team, patients are typically enrolled in a 6-12 week program that includes exercise training, psychosocial support, nutritional improvement, and education. Numerous studies (see Chest reference) have shown improvement in exercise capacity, better quality of life, and decreased hospitalizations. However, mortality is unchanged.
Lung volume reduction surgery (LVRS)
Ann Thorac Surg. 2006 Aug;82(2):431-43.
LVRS is a surgical option that improves survival in COPD patients with upper-lobe emphysema and low post-rehabilitation exercise capacity. The procedure involves resecting parts of the diseased lung. This has two main physiologic effects: (1) it reduces hyperinflation, allowing the diaphragm to contract at its optimal strength, and (2) it improves lung recoil because the inelastic portions of the lung are resected.