Definition
- Dev Med Child Neurol Suppl. 2007 Feb;109:8-14.
- Cerebral palsy (CP) is a heterogeneous group of movement disorders with various etiologies.
- The primary functional difficulty is in movement and posture, i.e. the movement disorder is not secondary to another neurofunctional disability.
- CP is associated with a permanent, non-progressive pathology that formed in utero or early infancy (before 2-3 years of age).
- CP excludes transient disease processes.
- CP is often accompanied by disturbances of sensation, perception, cognition, communication, behaviour, epilepsy, and secondary musculoskeletal problems.
Etiology
- Clin Obstet Gynecol. 2008 Dec;51(4):775-86.
- Pediatr Neurol. 2009 Mar;40(3):168-74.
- Essentials of Obstetrics and Gynecology, 5E (Hacker)
- Nelson Textbook of Pediatrics, 18E
- CP affects approximately 2-2.5/1000 live births in the Western world, and more children in the developing world.
- The ratio of affected males to females is 1.4:1.
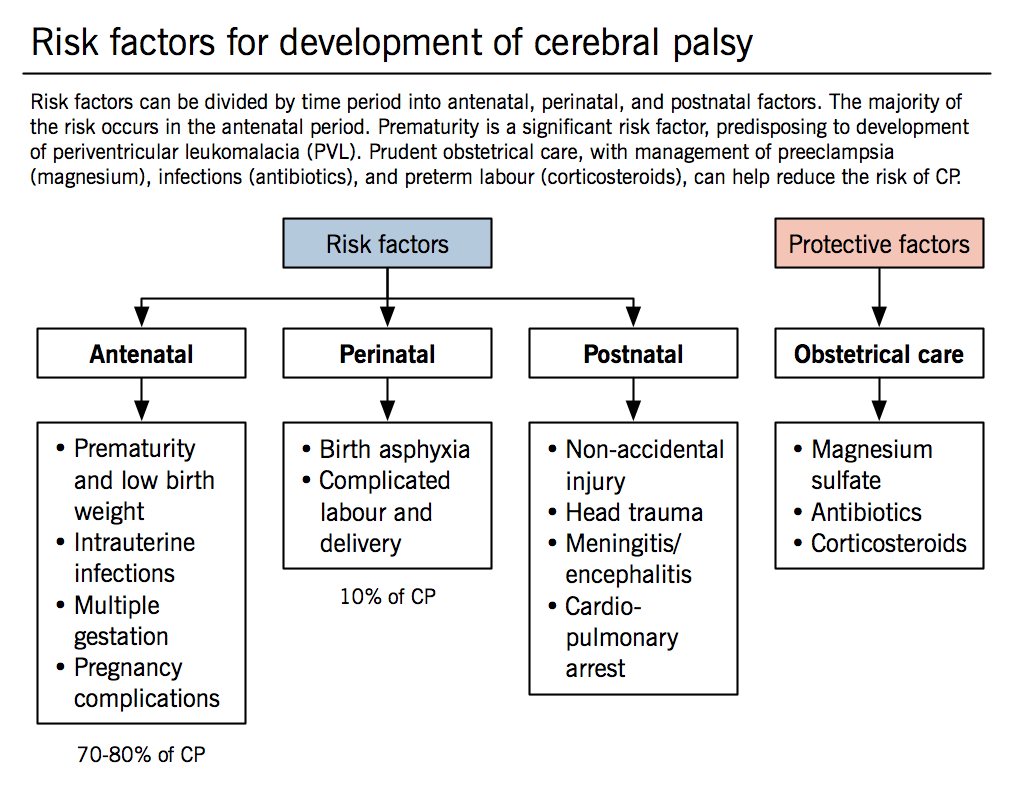
Antenatal (~70-80% of causes)
Prematurity and low birth weight
- Greater risk of CP with preterm deliveries (but since most deliveries happen close to term, most infants with CP (75%) are born after 36 weeks).
- There is a U-shaped association between CP and gestational age, where incidence of CP is increased in both preterm and postterm babies. The mechanism may be related to the physiological changes that trigger labour. Parturition is hypothesized to be partially related to fetal brain maturity, as fetuses with cerebral abnormalities tend to be delivered either preterm or postterm.
JAMA. 2010 Sep 1;304(9):976-82. - Periventricular leukomalacia (PVL) is a condition of underdeveloped white matter in the brain surrounding the ventricles. It is the leading cause of CP in preterm infants. PVL is discussed in the Pathophysiology section below.
- Intraventricular hemorrhage (IVH) is predominantly associated with prematurity and is due to fragility of developing blood vessels in the infant’s brain. IVH may cause PVL or ischemia in other parts of the brain. See Pathophysiology for details.
- There is a U-shaped association between CP and gestational age, where incidence of CP is increased in both preterm and postterm babies. The mechanism may be related to the physiological changes that trigger labour. Parturition is hypothesized to be partially related to fetal brain maturity, as fetuses with cerebral abnormalities tend to be delivered either preterm or postterm.
Infections
- Fetoplacental and uterine infection or inflammation can cause initiation of preterm labour, which can lead to CNS injury and CP. Underdeveloped fetal brains are more susceptible to inflammation and inflammatory cytokines. These cytokines are hypothesized to be responsible for the development of PVL.
- Chorioamnionitis is an infection of the chorion and amnion, the two membranes surrounding the developing fetus. It is the most frequently associated maternal infection in CP.
- TORCHS is an acronym for perinatal infections: toxoplasmosis, other infections (varicella zoster, adenovirus, enterovirus), rubella, cytomegalovirus, herpes simplex virus, syphilis. TORCHS infections are associated with approximately 5% of all CP cases.
Multiple gestation
- Increases the risk of antenatal complications, such as preterm labour, growth restriction, low birth weight, and death of a co-twin.
- Death of a co-twin in utero has been shown to induce neuropathologic changes that can lead to CP in the surviving twin. Prevalence of CP in the surviving twin was found to be 15x higher than average.
- Twinning is the single strongest risk factor for the development of CP.
Pregnancy complications in the mother
- Thrombophilias can lead to placental vascular injury and clotting of the fetal vessels.
- Hemorrhage and preeclampsia (placental abruption, placenta previa, and other causes of third trimester bleeding) seem to lead to premature delivery, conferring the same risks for CP as a premature infant according to some evidence.
Perinatal
- Birth asphyxia (~10%) is commonly associated with CP.
- CP is associated with complicated labour and delivery, but there is not a clear association between CP and the quality of perinatal care.
- Despite the advancement of prenatal and obstetrical care in the past 30 years, the incidence of CP has remained constant. This may be due to increased survival rates of premature and low birth weight babies.
Postnatal
- Non-accidental injury
- Head trauma
- Meningitis/encephalitis (including cerebral malaria in the developing world)
- Cardiopulmonary arrest
Obstetrical care (protective factors)
- Magnesium sulfate (used for tocolysis for preterm labour, and to increase the seizure threshold in mothers with preeclampsia) may reduce the risk of CP according to some studies, but further research is needed before it is used specifically as a neuroprotective agent for preterm births.
- Antibiotics used to treat bacterial vaginosis may reduce the rate of preterm delivery. In women with premature rupture of membranes, antibiotics reduce the risk of chorioamnionitis.
- Corticosteroids reduce the risk of CP, as steroids inhibit cytokine production, thus preventing PVL.
Pathophysiology
Arch Dis Child Fetal Neonatal Ed. 2008 Mar;93(2):F153-61.
Pediatric Ophthalmology: Current Thought and a Practical Guide, 1E (Wilson)
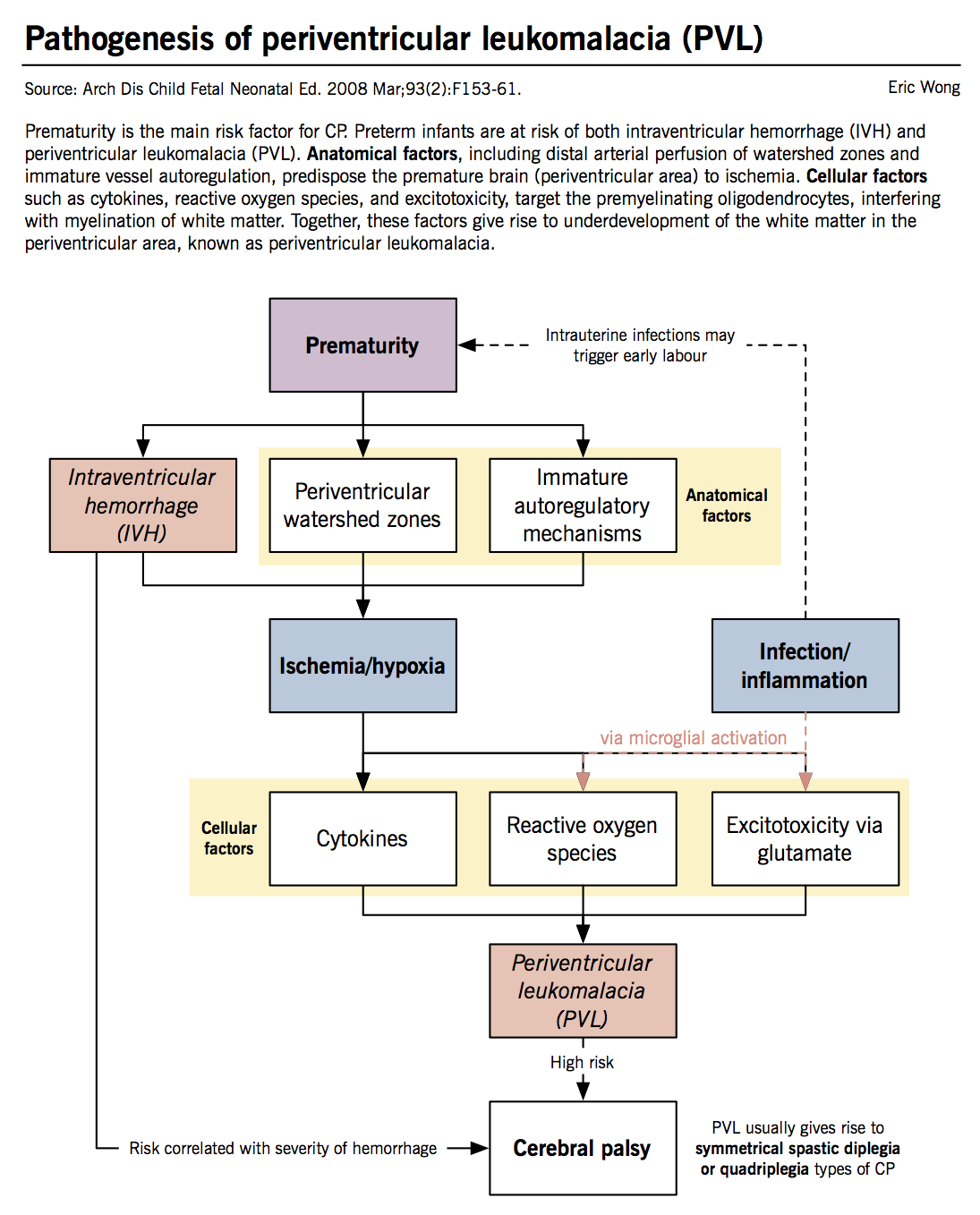
Preterm infants
The premature neonatal brain is susceptible to two main pathologies: intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL). Although both pathologies increase the risk of CP, PVL is more closely related to CP and is the leading cause in preterm infants. The term PVL describes white matter in the periventricular region that is underdeveloped or damaged (“leukomalacia”). Both IVH and PVL cause CP because the corticospinal tracts, composed of descending motor axons, course through the periventricular region.
Intraventricular hemorrhage (IVH)
IVH describes bleeding from the subependymal matrix (the origin of fetal brain cells) into the ventricles of the brain. The blood vessels around the ventricles develop late in the third trimester, thus preterm infants have underdeveloped periventricular blood vessels, predisposing them to increased risk of IVH. The risk of CP increases with the severity of IVH.
Periventricular leukomalacia (PVL)
IVH is a risk factor for PVL, but PVL is a separate pathological process. The pathogenesis of PVL arises from two important factors: (1) ischemia/hypoxia and (2) infection/inflammation.
Ischemia/hypoxia: The periventricular white matter of the neonatal brain is supplied by the distal segments of adjacent cerebral arteries. Although collateral blood flow from two arterial sources protects the area when one artery is blocked (e.g., thromboembolic stroke), this watershed zone is susceptible to damage from cerebral hypoperfusion (i.e., decreased cerebral blood flow in the brain overall). Since preterm and even term neonates have low cerebral blood flow, the periventricular white matter is susceptible to ischemic damage. Autoregulation of cerebral blood flow usually protects the fetal brain from hypoperfusion, however, it is limited in preterm infants due to immature vasoregulatory mechanisms and underdevelopment of arteriolar smooth muscles.
Infection and inflammation: This process involves microglial (brain macrophage) cell activation and cytokine release, which causes damage to a specific cell type in the developing brain called the oligodendrocyte. The oligodendrocytes are a type of supportive brain cell that wraps around neurons to form the myelin sheath, which is essential for white matter development. Intrauterine infections activate the fetal immune system, which produces cytokines (e.g., interferon γ and TNF-α) that are toxic to premyelinating oligodendrocytes. Infections also activate microglial cells, which release free radicals. Premyelinating oligodendrocytes have immature defences against reactive oxygen species (e.g., low production of glutathione, an important antioxidant). IVH is hypothesized to cause PVL because iron-rich blood causes iron-mediated conversion of hydrogen peroxide to hydroxyl radical, contributing to oxidative damage.
Excitotoxicity is a process where increased extracellular glutamate levels stimulate oligodendrocytes to increase calcium influx, which stimulates reactive oxidative species release. Glutamate is increased because hypoxia causes white matter cells to reduce reuptake of glutamate due to lack of energy to operate glutamate pumps. Glutamate is also released from microglial cells during the inflammatory response.
Term infants
- Circulation and autoregulation of cerebral blood flow are similar to that of an adult in a full term infant. Ischemic and hemorrhagic injuries tend to follow similar patterns of those in adults:
- Watershed areas where the three major cerebral arteries end in the cortex. This is the most common area of injury.
- Basal ganglia damage can cause extrapyramidal or dyskinetic CP.
Clinical features
- Nelson Textbook of Pediatrics, 18E
- Eur J Neurol. 2002 May;9 Suppl 1:3-9; discussion 53-61.
- Neuroscience, 3E (Purves)
- Clinical Neuroanatomy, 26E (Waxman)
- Rosenbaum P, Rosenbloom L (2012). Cerebral Palsy. From Diagnosis to Adult Life. London: Mac Keith Press.
The clinical features of neurological disorders depend on the location of damage to the nervous system. The location of damage can be divided into upper motor neuron or lower motor neuron. The pathology in CP is in the upper motor neurons.
Upper motor neuron (UMN)
- Includes neurons in the brain and spinal cord (central nervous system, CNS) that control movement of muscles. UMN synapse onto lower motor neurons at the ventral horn of the spinal cord at the level which the neuron leaves the cord. Upper motor neurons travel through the pyramidal tracts (i.e., corticospinal tracts).
- UMN lesions can cause positive or negative signs:
- Positive signs include muscle overactivity and spasticity, generally due to reduced descending inhibitory signals from the brain.
- Negative signs include weakness or loss of dexterity, generally due to reduced descending excitatory signals from the brain.
- Note that lesions in the extrapyramidal tracts do not cause these UMN signs. The extrapyramidal tracts link the cerebellum and basal ganglia with LMN. They function to modulate and refine movement rather than directly cause movement, unlike the upper motor neurons in the pyramidal tracts.
- Spasticity is defined as a velocity-dependent increase in the tonic stretch reflex. It is characteristic of an UMN lesion where there is disturbance of the supraspinal excitatory and inhibitory neurons, leading to a net disinhibition of the spinal reflexes.
- Tonic stretch reflex: Normally, passively stretching a muscle group (e.g., stretching the biceps by passively extending the elbow) causes contraction of the same muscle group to prevent overstretching and injury. This tonic stretch reflex is a spinal reflex (i.e., it does not require input from the brain). This reflex is normally minimal or not present.
- Spasticity: Injury to descending UMNs that usually provide inhibitory signals to the spinal reflexes causes net disinhibition, which increases muscle tone (contraction) as the muscle is passively stretched. The faster the velocity of stretching, the stronger the reflex.
Lower motor neuron (LMN)
- Includes neurons from ventral horn of the spinal cord grey matter that exit the spinal cord and attach to skeletal muscles. The motor nuclei of cranial nerves in the brainstem are also lower motor neurons because they directly attach to muscles in the head and neck.
- LMNs relay signals from the UMNs to skeletal muscles to initiate excitation-contraction coupling, allowing individual units of a muscle to contract in a synchronized manner.
- Their function is to provide muscle tone to skeletal muscles. In LMN lesions, there is no neural input to muscles, which leads to flaccid paralysis due to lack of resting muscle tone and subsequent atrophy from disuse. The lack of excitation-contraction coupling causes fasciculations in the muscles, where individual sacromeres (contractile units in muscles) fire and contract at random.
Comparison of UMN and LMN lesion clinical presentations
| Upper motor neuron lesion | Lower motor neuron lesion |
| Spasticity
Increased tone Hyperactive deep reflexes Clonus Babinski sign Little to no muscle atrophy |
Flaccid paralysis
Decreased or absent deep tendon reflexes Fasciculations and fibrillations Severe muscle atrophy (from disuse) |
Common clinical presentations
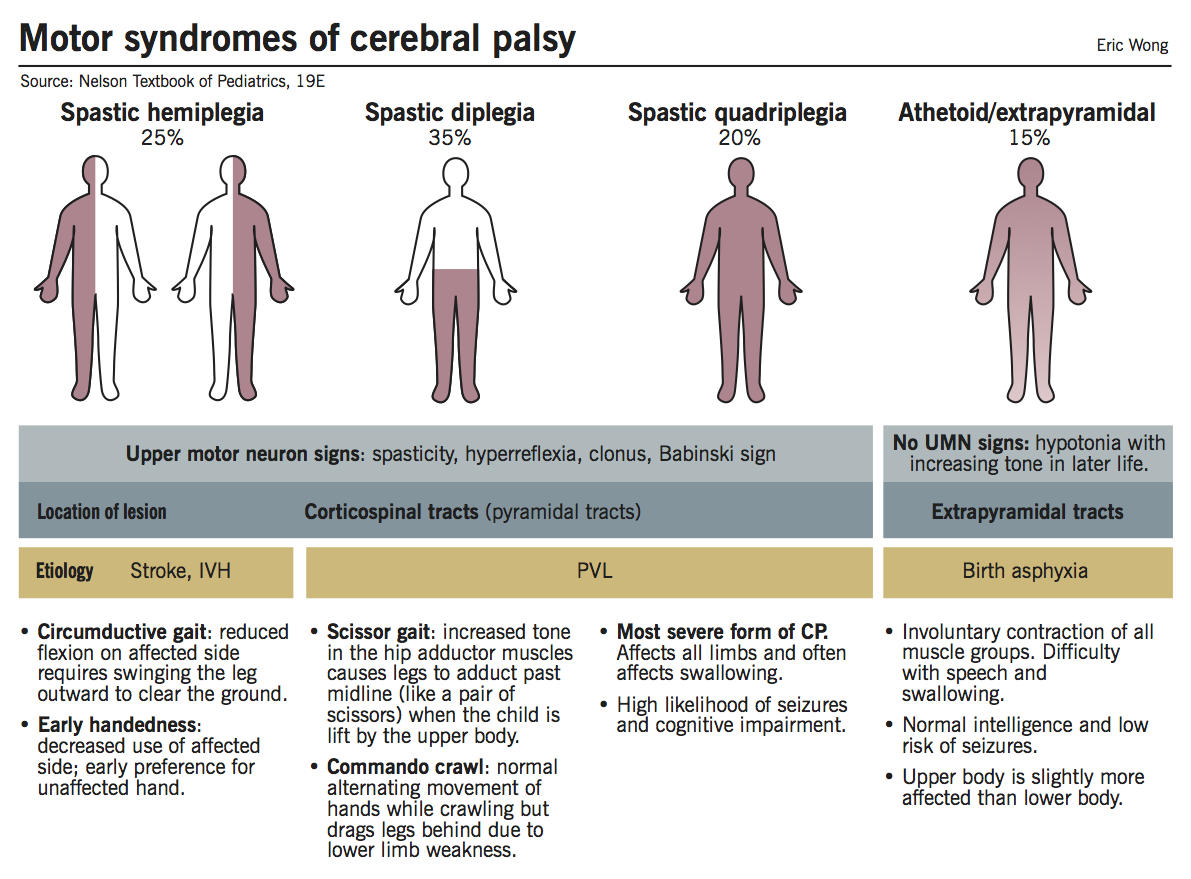
Spastic hemiplegia
- Affects one side of the body more than the other (though both sides may be affected).
- Upper limb affected more than lower.
- Hand preference obvious at an early age.
- Delayed walking (18-24 months) with a circumductive gait.
- Circumductive gait: one leg is stiff and upon stepping it is rotated away from the body, and then towards it (i.e., a semicircle shape). The stiffness in the affected leg limits flexion and the patient has to raise the pelvis to swing the leg out to lift the leg enough to clear the ground.
- On exam:
- Circumductive gait.
- On affected side:
- Growth differences of hand and thumbnail.
- Tiptoe walking on one side, due to increased tone in the gastrocnemius muscles. Spasticity has a greater effect on the postural (antigravity) muscles, e.g., gastrocnemius and sartorius.
- Unilateral ankle clonus (usually).
- Babinski sign.
- Brisk deep tendon reflexes.
- Weakness of hand and foot dorsiflexors.
- Seizure disorder presenting before 2 years of age in 1/3 of children. Cognitive abnormalities in 25% of cases.
Spastic diplegia
- Bilateral spasticity of the limbs with legs more affected than arms.
- First clinical signs appear around the time when the child starts to crawl.
- Commando crawl: the child uses arms in a normal reciprocal manner but drags legs behind rather than using legs as well.
- On exam:
- Spasticity of the legs.
- Brisk reflexes.
- Ankle clonus.
- Bilateral Babinski sign.
- Scissoring posture of legs when held in the air supported by the axillae due to spasticity in the hip adductor muscles.
- Tiptoe walking.
- Atrophy and impaired growth of legs in severe cases.
- Strongly associated with white matter damage in utero between 20-34 weeks of gestation.
- Most common neuropathologic feature is PVL.
- Minimal risk of seizure disorder.
- Normal intellectual development is common, but many children still have learning disabilities.
- Other deficits in sensory areas, like vision, may be present.
Spastic quadriplegia (‘total body involvement’)
- Most severe form of CP.
- Motor impairment to all extremities.
- High association with cognitive deficiencies and seizure disorders.
- Increased difficulty swallowing due to supranuclear bulbar palsies, which can cause the child to have aspiration pneumonias.
- Most common neuropathologic lesion is PVL, but may also include basal ganglia damage.
- On exam:
- Increased tone and spasticity in all limbs.
- Decreased spontaneous movements.
- Brisk reflexes and plantar extension responses.
- Flexion contractures of knees and elbows commonly present in late childhood.
- Delay in speech and the presence of visual abnormalities are common.
Athetoid (extrapyramidal, dyskinetic)
- Less common than spastic CPs.
- 15-20% of patients with CP.
- On exam:
- Infants are usually hypotonic with poor head control and head lag.
- Variably increased tone with rigidity and dystonia with age.
- Upper extremities more affected than lower extremities.
- Feeding and speech difficulties due to affected oropharyngeal muscles.
- Seizure disorders are uncommon.
- Normal intellectual development in many patients.
- Most commonly associated with birth asphyxia.
- Neuropathologic lesions of the basal ganglia and thalamus (signals are relayed by the extrapyramidal tracts) are most common.
- Other causes: kernicterus (now rare in the Western world due to maternal blood group screening and immunization against Rh factor), metabolic genetic disorders.
Diagnosis
- Pediatr Neurol. 2010 Mar;42(3):177-80.
- Nelson Textbook of Pediatrics, 18E.
- Rosenbaum P, Rosenbloom L (2012). Cerebral Palsy. From Diagnosis to Adult Life. London: Mac Keith Press.
|
CP is essentially a clinical diagnosis – there are no pathognomonic signs or diagnostic tests. |
General assessment
- Suspicions of CP are commonly based on a positive history of adverse perinatal or antenatal events.
- If no positive history, suspicions are often raised by parental or family observations of developmental delays.
- Observations of the child while being held by their caregiver include: movements, posture, dysmorphic features, etc.
- Growth curves: crossing major percentile lines raises concerns for growth and developmental disorder
- CP is non-progressive but can change its clinical manifestations throughout childhood. Therefore, such changes are important to discuss with parents.
- Examine and rule out the possibility of degenerative diseases, metabolic disorders, spinal cord lesions/tumours, muscular dystrophy, and anomalies of the cervical spinal cord and skull.
Functional assessment
- It can be helpful to refer to the Gross Motor Function Classification System (GMFCS), which is a 5-level classification scheme used to evaluate gross motor function in children with CP.
- Specific attention to head posture, head control, ability to sit independently, and presence of independent mobility is important.
- When independent mobility is present, gait, asymmetry, and abnormalities of posture should be assessed.
- Assessing chewing abilities or oromotor functioning is also important in determining any safety concerns with regards to feeding.
- Assessing speech production and clarity.
Characteristic features of CP based on age*
| Age | Characteristics that may indicate CP |
| < 6 months |
|
| > 6 months |
|
| > 10 months |
|
Brain imaging is one of the most useful diagnostic tools
- Confirmation of brain/spinal cord lesion via MRI.
- Location and extent of lesion.
- Can at times be ‘normal’ in the face of clear clinical findings.
Additional tests
- Hearing and vision testing
- Genetic screening in those with congenital malformations or if evidence of a genetic disorder.
- Tests for thrombophilia in those where a stroke is the suspected cause.
* It is very important to differentiate whether a delay or difference constitutes an abnormality, and the clinician must do so by continuing to reassess children as they develop. The time for reassessment is dependent on the normal achievement of specific developmental milestones.
Management
- Nelson Textbook of Pediatrics, 18E.
- Rosenbaum P, Rosenbloom L (2012). Cerebral Palsy. From Diagnosis to Adult Life. London: Mac Keith Press.
- Children with CP often have multiple developmental issues that are best managed by a multidisciplinary team of health care professionals.
- Child Development Teams act as excellent liaisons between the different health care professionals, and are able to provide a structured program for treatment, suitable to each child’s needs.
- Health care professionals usually involved in the care of children with CP include:
- Developmental pediatricians
- Monitor and promote the child’s development.
- Connect with other health care professionals as needed.
- Support children and families with the patient’s development in the context of their individual family and community.
- Occupational therapists
- Implement the use of assistive devices (e.g., wheelchairs, ankle-foot orthosis (AFOs), walkers, appropriate toys, and adaptations) that can be made to the home to accommodate the child.
- Speech therapists
- Assist with feeding, as these children often have difficulties with chewing and swallowing.
- The development of speech language and the provision of non-verbal communication systems as necessary.
- Physiotherapists
- Assist with the development of muscle control, overcoming weakness, minimizing spasticity, and preventing contractures.
- Nutritionists
- Malnutrition may be seen in children with feeding difficulties.
- Food must be given in a form that the child is able to chew and swallow.
- Energy-rich supplements may be needed.
- Enteral feeding may also be necessary if oral intake is insufficient to maintain nutrition via surgical placement of G-tube or GJ-tube.
- Orthopedic surgeons
- Chronic muscle weakness or spasticity can cause orthopedic deformities that need surgical correction, e.g., dislocation of the hips due to spasticity of the thigh adductors, deformity of the ankle from calf muscle spasticity.
- Developmental pediatricians
Prognosis
- Clin Obstet Gynecol. 2008 Dec;51(4):816-28.
- JAMA. 2002 Sep 18;288(11):1357-63.
- Pediatrics. 2011 Aug;128(2):e299-307.
- Dev Med Child Neurol. 2008 Jul;50(7):487-93.
- The use of the GMFCS (in the Ontario Motor Growth Study) has been shown to be an effective tool in assessing outcomes for individuals with CP.
- Motor assessments have been used alongside growth charts to characterize gross motor development over time.
- These trends can be divided into 5 distinct motor development curves which children can be categorized into to assist with providing further prognostic information for parents.
- Prognosis for motor function depends on the type and severity of motor impairment.
- Individuals with CP on average have a life expectancy that is 44% of normal (this can be applied to countries with varying life expectancy rates).
- Mortality risk increases with increasing number of impairments (e.g., intellectual, hearing, vision).
- Research has shown that the strongest predictors of early mortality are immobility and impaired feeding ability (i.e., the need for tube feeding).
- Shortest life expectancy is associated with individuals who are unable to lift their head in prone position.