Definitions
Heart. 2000 Mar;83(3):361-6.
N Engl J Med. 2005 Jun 16;352(24):2524-33.
Lilly, Pathophysiology of Heart Disease, 2007.
Ischemic heart disease: a condition in which imbalance between myocardial oxygen supply and demand, most often caused by atherosclerosis of the coronary arteries, results in myocardial hypoxia and accumulation of waste metabolites.
Acute coronary syndromes (ACS): life-threatening conditions that encompass a continuum ranging from unstable angina (UA) pectoris to the development of a large acute myocardial infarction (MI), a condition of irreversible myocardial necrosis.
Mechanisms of ischemia
Myocardial ischemia is a consequence of reduced blood flow in coronary arteries, due to a combination of fixed vessel narrowing and abnormal vascular tone as a result of atherosclerosis and endothelial dysfunction. This leads to an imbalance between myocardial oxygen supply and demand.
Fixed vessel narrowing
Several factors affect the hemodynamic significance of a stenotic lesion:
- Length of the lesion and more importantly the degree of vessel narrowing that it causes
- i.e. the more a stenosis narrows the lumen diameter, the higher the resistance to flow
- Amount of compensatory vasodilatation that smaller, distal resistance vessels (which are less susceptible to flow-limiting plaques) are able to achieve
- Distal coronary vessels can act as a reserve by adjusting their vasomotor tone in response to metabolic needs
- Myocardial oxygen demand
- At rest – full dilatation of the resistance vessels may be sufficient to compensate for a narrowed diameter and provide adequate blood flow to meet oxygen requirements
- During exercise – when oxygen demand increases from the elevated heart rate and force of contraction, coronary reserve may not be enough to compensate if a proximal stenosis is sufficiently severe, and distal ischemia ensues
Endothelial dysfunction
In normal endothelium, the balance of vasoactive mediators favours vasodilatation and a net antithrombotic effect. In atherosclerotic arteries, however, endothelial dysfunction contributes to myocardial ischemia in the following ways:
- Inappropriate vasoconstriction of coronary arteries
- Impaired release of endothelial vasodilators (e.g. NO, prostacyclins) such that the constrictor effect of catecholamines on arterial smooth muscle during stress and exercise cannot be outweighed
- Resultant decrease in coronary blood flow contributes to ischemia
- In the context of plaque rupture and thrombus formation, dysfunctional endothelium is unable to release NO in response to platelet products (serotonin, ADP)
- Vasoconstricting actions of platelet products thus predominate, and flow through the arterial lumen is further compromised
- Impaired release of endothelial vasodilators (e.g. NO, prostacyclins) such that the constrictor effect of catecholamines on arterial smooth muscle during stress and exercise cannot be outweighed
- Loss of normal antithrombotic properties
- Endothelial vasodilators such as NO and prostacyclins also have an antithrombotic effect which is greatly reduced when their release is impaired
Non-atherosclerotic causes of ischemia
The following conditions also result in an imbalance between myocardial oxygen supply and demand and can lead to ischemia:
- Decreased coronary perfusion pressure due to hypotension (e.g. hypovolemia, septic shock)
- Decreased blood oxygen content (e.g. marked anemia, pulmonary disease)
- Significant increase in myocardial oxygen demand (e.g. rapid tachycardias, acute hypertension, severe aortic stenosis)
- Unusual coronary abnormalities leading to decreased perfusion of the myocardium
- Coronary emboli from endocarditis or artificial heart valves
- Inflammation of the coronary arteries from vasculitic syndromes
- Severe transient coronary spasm, either primary or induced by cocaine abuse
- Congenital abnormalities, trauma or aneurysm of the coronary arteries
Consequences of ischemia on the myocardium
The consequences of ischemia reflect the inadequate myocardial oxygenation and local accumulation of metabolic waste products.
Ultimately, the severity and duration of imbalance between oxygen supply and demand will determine the fate of the myocardium. The spectrum of myocardial dysfunction ranges from rapid and full recovery of myocyte function (typically after a brief episode of stable angina) to prolonged contractile dysfunction without myocyte necrosis with potential recovery of normal function (i.e. stunned myocardium, hibernating myocardium) and ultimately irreversible myocardial necrosis (i.e. myocardial infarction).
Stunned myocardium
- Tissue that demonstrates prolonged systolic dysfunction even after the return of normal myocardial blood flow
- Magnitude of stunning is proportional to the degree of the preceding ischemia
- Delayed recovery of function is due to myocyte calcium overload and accumulation of oxygen-derived free radicals during the ischemia
- Abnormalities resulting from ischemia are reversible and contractile function gradually recovers
Hibernating myocardium
- Tissue that manifests chronic ventricular contractile dysfunction in response to a persistently reduced blood supply, usually in the context of multivessel coronary artery disease
- Damage is reversible and ventricular function can promptly improve if appropriate blood flow is restored
Myocardial infarction
- Myocyte necrosis as a result of prolonged myocardial ischemia
- Amount of tissue that succumbs to infarction depends on:
- Mass of myocardim perfused by the occluded vessel
- Magnitude and duration of impaired coronary blood flow
- Oxygen demand of the affected region
- Adequacy of collateral vessels that provide blood supply from neighbouring nonoccluded coronary vessels
- Degree of tissue response to the ischemic process
Ischemic syndromes
Distinct clinical syndromes can result based on the timing and severity of a myocardial ischemic insult.
1. Stable angina
- Pattern of chronic, predictable, transient angina during exertion or emotional stress
- Generally caused by a fixed obstructive atherosclerotic plaque in one or more coronary arteries, in addition to inappropriate vasoconstriction resulting from atherosclerosis-associated endothelial dysfunction
- Severity of symptoms usually related to degree of stenosis and compensatory capacity of distant resistance vessels to vasodilate
2. Unstable angina (also part of acute coronary syndromes, see below)
- Represents an acceleration of symptoms from stable angina, such as a sudden increase in the rate and duration of ischemic episodes, occurring with lesser degrees of exertion and sometimes even at rest
- Can be a precursor to an acute myocardial infarction
- Underlying pathophysiologic mechanism typically involves rupture of an unstable atherosclerotic plaque with subsequent platelet aggregation and thrombosis
3. Variant angina
- Episode of focal coronary artery spasm in the absence of overt atherosclerotic lesions
- Mechanism leading to intense vasospasm is not completely understood but thought to involve increased sympathetic activity in combination with endothelial dysfunction
- Often occurs at rest since ischemia results from a transient reduction in coronary oxygen supply rather than an increased myocardial oxygen demand
4. Silent ischemia
- Episode of cardiac ischemia that occurs in the absence of perceptible discomfort or pain
- Can occur in patients who experience typical symptomatic angina, or be the only manifestation of coronary artery disease
- Pathophysiology is unknown, but thought to involve impaired pain sensation resulting from peripheral neuropathy, since silent ischemia is particularly common among patients with diabetes
5. Syndrome X
- Refers to patients with typical symptoms of angina pectoris who have no evidence of significant coronary artery disease on angiograms
- Thought to be due to inadequate vasodilator reserve of coronary resistance vessels, which do not dilate appropriately during periods of increased myocardial oxygen demand
Acute coronary syndromes (ACS)
Life-threatening conditions that encompass a continuum ranging from unstable angina pectoris to large acute myocardial infarctions with irreversible necrosis of heart muscle.
The type of ACS that ensues depends on the degree of coronary obstruction and associated ischemia:
|
|
Unstable angina (UA) |
Non-ST-elevation myocardial infarction (NSTEMI) |
ST-elevation myocardial infarction (STEMI) |
|
Thrombus size |
partially occlusive |
partially occlusive |
completely occlusive |
|
Myocardial necrosis |
absent |
present in small amount |
present in large amount |
|
Serum biomarkers (cTnT, cTnI, CK-MB) |
absent |
present |
present |
Common initiating pathophysiologic mechanism:
- Disruption of an atherosclerotic plaque (i.e. plaque rupture) with subsequent platelet aggregation and formation of an intracoronary thrombus
- Primary hemostasis – circulating platelets adhere to collagen in the exposed vascular subendothelium and aggregate to form a platelet plug
- Secondary hemostasis – exposed subendothelial tissue factor triggers the coagulation cascade to stabilize and strengthen the platelet plug into a fibrin clot
- Endothelial dysfunction in the atherosclerotic artery leads to inappropriate vasoconstriction and loss of normal antithrombotic defenses which overwhelm endogenous protective antithrombotic mechanisms
- Previously narrowed atherosclerotic coronary artery becomes severely or completely occluded by the emerging thrombus
- Resulting impairment in blood flow causes a marked imbalance between myocardial oxygen supply and demand
Factors predisposing to plaque rupture
- Intrinsic stability of the atherosclerotic lesion
- A plaque that is compromised and weakened by internal chemical processes or that has a thinner fibrous cap will be more subject to rupture
- Physical stresses on the atherosclerotic lesion
- Key physiologic stressors include increased blood pressure, heart rate and force of ventricular contraction
- Can be promoted by activation of the sympathetic nervous system in the context of strenuous physical activity or emotional upset
Pathophysiology of the ischemic process
Early changes in infarction (minutes to days)
- Drop in tissue oxygen levels
- Rapid conversion from aerobic to anaerobic metabolism
- Impaired glycolysis and ATP production → impaired contractile protein function
- Systolic dysfunction – loss of synchroneous myocyte contraction → compromised cardiac output
- Diastolic dysfunction – reduced ventricular compliance (i.e. impaired relaxation) and elevation of ventricular filling pressures
- Accumulation of lactic acid and reduction in pH
- Impairment of transmembrane Na-K-ATPase due to impaired ATP production
- Increased intracellular Na → intracellular edema
- Increased extracellular K → alteration in transmembrane potential → electrical instability and susceptibility to arrhythmias
- Increased intracellular Ca → activation of degradative lipases and proteases → tissue necrosis
- Acute inflammatory response with infiltration of neutrophils leading to further tissue damage
Late changes in infarction (days to weeks)
- Resorption of irreversibly injured/dead myocytes by macrophages
- Structural weakness of ventricular wall and susceptibility to myocardial wall rupture
- Fibrous tissue deposition and scarring
- Ventricular remodeling
- Infarct expansion – thinning and dilatation of necrotic tissue without additional necrosis
- Increased ventricular wall stress
- Further impairment in systolic contractile function
- Increased likelihood of aneurysm formation
- Remodeling of non-infarcted ventricle
- Dilatation of overworked non-infarcted segments subjected to increased wall stress
- Enlargement initially compensatory to increase cardiac output via Frank-Starling mechanism, but can eventually predispose to ventricular arrhythmias and lead to heart failure
- Infarct expansion – thinning and dilatation of necrotic tissue without additional necrosis
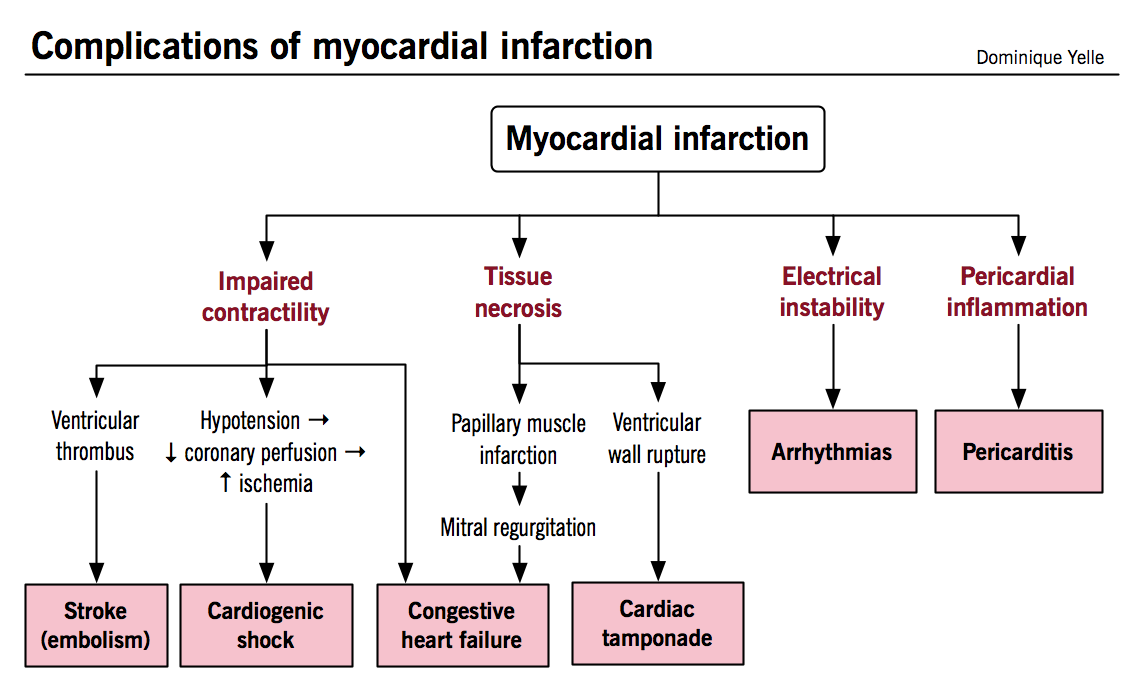
Complications of myocardial infarction
Early complications result mainly from myocardial necrosis itself, while later complications reflect the inflammation and healing of necrotic tissue.
Arrhythmias
- In addition to arising from the electrical instability of ischemic myocardium, they can also be caused by interruption of perfusion to structures of the conduction pathway (i.e. SA node, AV node, bundle branches)
Congestive heart failure
- Can be directly caused by impaired contractility resulting in both systolic and diastolic dysfunction
- More rarely results from papillary muscle infarction or rupture causing moderate to severe mitral regurgitation
Thromboembolism
- Intra-ventricular thrombus formation can arise from stasis of blood flow in regions of impaired LV contraction, especially when the infarction involves the apex or when an aneurysm has formed
Pericarditis
- Can arise in the early post-MI period when necrosis and neutrophilic infiltrates extend from the myocardium to involve the adjacent pericardium
Ventricular aneurysm
- Develops as the ventricular wall is weakened but not perforated by the phagocytic clearance of necrotic tissue
Cardiac tamponade
- Hemorrhage into the pericardial space due to ventricular free wall rupture (structurally weakened by necrosis) leads to rapid filling of the pericardial space and severe restriction of ventricular filling; often lethal
Cardiogenic shock
- Severely decreased cardiac output and hypotension with inadequate perfusion of peripheral tissues develops when more than 40% of the LV mass is infarcted
- Self-perpetuating mechanism whereby impaired contractility results in hypotension, decreased coronary perfusion, exacerbation of ischemic damage, further decrease in contractile function, and so forth
Clinical presentation of myocardial ischemia
|
Symptoms |
Mechanism |
|
Chest pain/discomfort (typically retrosternal, “pressure”, “discomfort”, “tightness”, “burning” or “heaviness”) |
Metabolic products such as lactate, serotonin and adenosine accumulate locally and may activate peripheral pain receptors in the C7 to T4 distribution |
|
Tachycardia |
Dangerous arrhythmias can be precipitated by transient abnormalities of myocyte ion transport and accumulation of local metabolites; increased sympathetic tone during myocardial ischemia can also account for an increased heart rate |
|
Dyspnea |
Transiently impaired LV relaxation leads to elevation of LV diastolic pressure which is transmitted backwards into the pulmonary capillaries and can precipitate pulmonary congestion and symptoms of dyspnea |
|
Diaphoresis |
Increased sympathetic tone during the discomfort of an acute ischemic attack |
|
Nausea/vomiting |
Increased parasympathetic tone during the discomfort of an acute ischemic attack |
Treatment options and their role in the management of acute coronary syndromes
Circulation. 2004 Aug 31;110(9):e82-292 (updates in Circulation. 2008 Jan 15;117(2):296-329)
Circulation. 2007 Aug 14;116(7):e148-304
Therapy in ACS is targeted at providing measures that improve the balance between myocardial oxygen supply and demand, stabilizing the intracoronary thrombus that in initiated the syndrome and ultimately, restoring blood flow to the ischemic myocardium.
|
Treatment |
Underlying mechanism of action |
|
Immediate adjunctive treatment |
|
|
Nitrates |
Decrease anginal symptoms by inducing coronary vasodilation and improving myocardial O2 supply, and by decreasing myocardial O2 demand by decreasing preload through venodilatation |
|
Beta blockers |
Decrease myocardial O2 demand by decreasing heart rate and contractility; in addition, also contribute to electrical stability |
|
Calcium channel blockers |
Decrease myocardial O2 demand by decreasing heart rate and contractility, decreasing wall stress via decreased blood pressure, and decreasing preload via venodilatation |
|
Morphine |
Reduces myocardial oxygen demands by decreasing chest pain and anxiety |
|
Oxygen |
Improves oxygen supply in patients with hypoxemia |
|
Antiplatelet therapy |
|
|
Aspirin |
Prevents further thrombus formation by inhibiting platelet synthesis of thromboxane A2, an important mediator of platelet activation |
|
Clopidogrel (or other ADP receptor blockers) |
Inhibit ADP-mediated activation of platelets, thereby preventing expansion of the existing thrombus; have superior outcomes when used in combination with Aspirin |
|
GP IIb/IIIa inhibitors |
Potent antiplatelet agents that block the final common pathway of platelet aggregation; often used in patients undergoing PCI as they are very effective in reducing cardiac events in these patients |
|
Anticoagulant therapy |
|
|
Unfractionated heparin (UFH) or low molecular weight heparin (LMWH) |
UFH an LMWH, which preferentially bind to antithrombin III and factor Xa, respectively, slow thrombin formation and impede clot development |
|
Fibrinolysis |
|
|
Recombinant tissue-type plasminogen activators (tPA, rPA and TNK-tPA) |
Transform the inactive precursor plasminogen into the active protease plasmin, which lyses fibrin clots, thereby accelerating lysis of the occlusive intracoronary thrombus and restoring blood flow |
|
Primary percutaneous coronary intervention (PCI) |
|
|
Plain old balloon angioplasty (POBA) |
Inflation of a balloon within a stenosed coronary artery mechanically dilates the affected vessel to restore blood flow, both by compressing the atherosclerotic plaque and stretching the underlying media |
|
Bare metal stents |
Mechanically maintain the patency of coronary arteries occluded by atherosclerotic plaques |
|
Drug-eluting stents |
In addition to maintaining patency, these stents release antiproliferative agents such as sirolimus or paclitaxel, which prevent neointimal proliferation (migration of smooth muscle cells and production of extracellular matrix), thereby decreasing the rate of in-stent restenosis |
|
Surgical revascularization |
|
|
Coronary artery bypass graft (CABG) |
Restores coronary blood flow by using a healthy patent artery to bridge circulation around an occlusive lesion within an atherosclerotic coronary vessel |
Approach to the management of patients with ACS
|
UA/NSTEMI |
All patients |
STEMI |
|
In high risk patients:
|
Additional therapies shown to have long-term outcome benefits
|
Reperfusion method selected based on timing:
|